Focuses On Professional Cleanroom Project And Pharmaceutical Cleanroom Equipment.

Customized Medical Device Manufacturing Isolator
After Warranty Service: Video technical support, Online support
Certification: CE ISO GMP
Name: VHP Isolator
Application: Cleanroom, lab, dust-free room
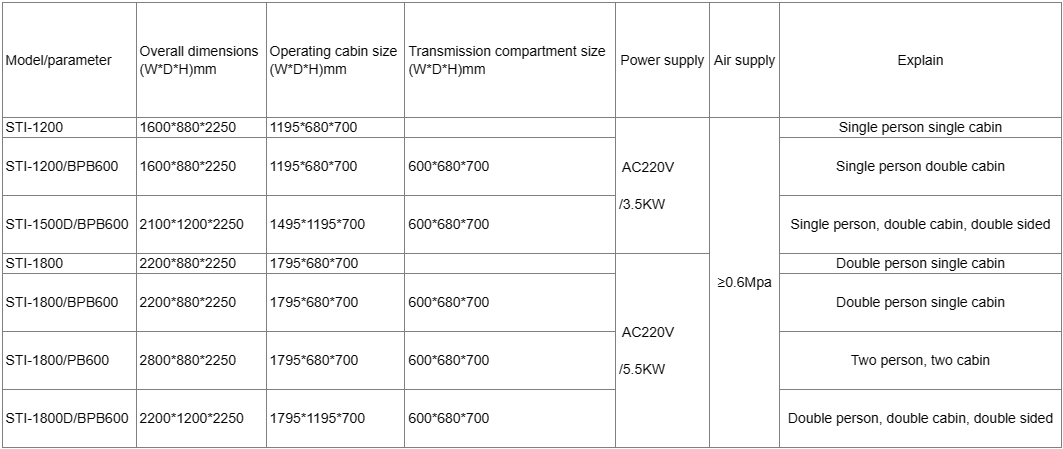
Compressed Air Volume: 0.5MPa
Power V/Kw: AC220 /3.5
Transfer Cabin Size (W*D*H)mm: 600*680*700
Operating Cabin Size(W*D*H)mm: 1195*680*700
Outer Dimension(W*D*H)mm: 1600*880*2250
Cabin Material: External Full 304 Stainless Steel
Limit Discounts
Description
The Customized Medical Device Manufacturing Isolator is designed to provide a sterile and controlled environment for the safe and efficient manufacturing of medical devices. This isolator ensures full compliance with GMP standards, allowing manufacturers to meet stringent regulatory requirements while minimizing contamination risks. Tailored to the specific needs of medical device production, this isolator offers high flexibility, precision control, and seamless integration with automated processes.
With advanced air filtration systems, robust material handling solutions, and customizable options for size, access ports, and automation, this isolator is ideal for creating a safe, controlled production environment for sensitive medical devices. Whether producing surgical instruments, implants, or diagnostic devices, the isolator guarantees protection from contaminants while ensuring product quality and operator safety.
Applications:
- Surgical Instrument Manufacturing: Ensures contamination-free production and sterilization of surgical tools.
- Implantable Device Production: Provides a controlled environment for manufacturing and assembling medical implants.
- Diagnostic Device Assembly: Supports precise, sterile conditions for producing diagnostic devices, such as catheters and test kits.
- Sterile Packaging: Ensures a contamination-free environment for packaging sterile medical devices.
- Customized Medical Device Production: Tailored solutions for manufacturing unique or complex medical devices requiring specialized handling and environmental control.
 | Key Features:
|
| Material | External all 304 stainless steel, internal chamber 316L stainless steel |
| Cleanliness level | Class A |
| Wind speed | 0.45m/s ± 20% |
| Leakage rate | ≤ 0.5% VOL/h (below 100pa) |
| Exhaust volume | ≥ 300m ³/ H |
| Pressure control range | 100pa~+100pa |
| Sterilization efficiency | 6lg |
| VHP residual removal efficiency | ≤ 1PPM |
| Control method | Manual or automatic |
| Noise | ≤ 68dB (A) |
| illumination | ≥ 350LUX |
Installation