Focuses On Professional Cleanroom Project And Pharmaceutical Cleanroom Equipment.

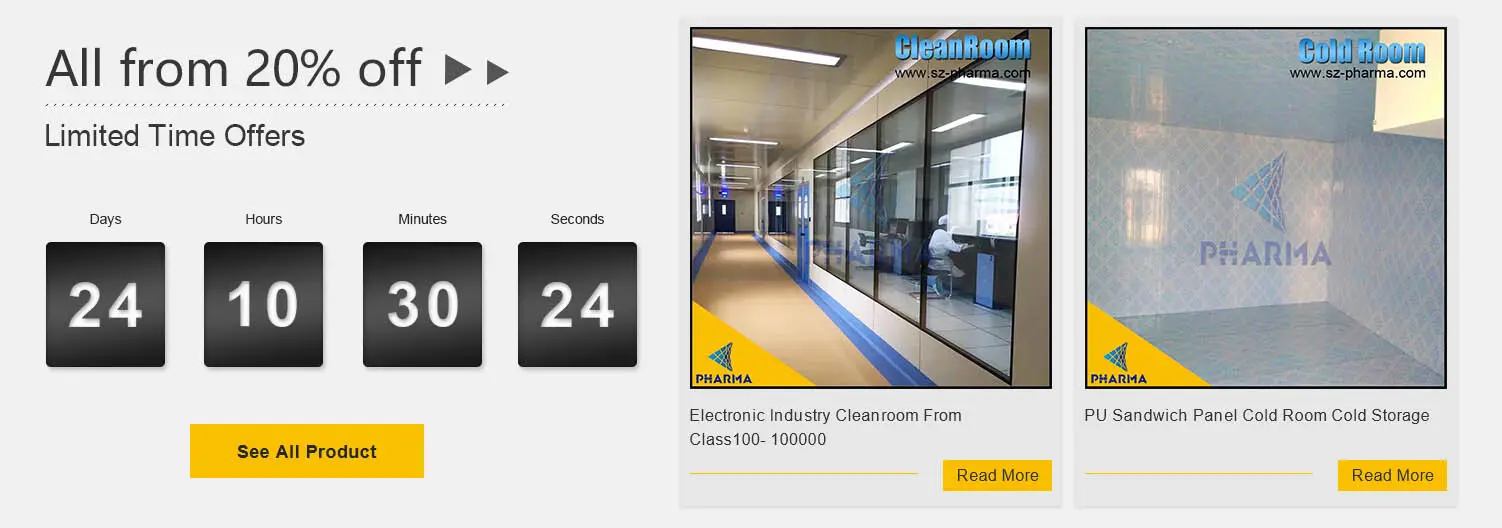

















PHARMA newly gmp cleanroom check now for electronics factory
After Warranty Service:Video technical support, Online support, Field maintenance and repair service
Warranty:1 year
Certification:CE ISO GMP
Usage:Hospital,Biopharmaceutical
Material:sandwich panels+ aluminum construction
Lighting:LED(Customizable quantity)
Clean grade:From Class100-10000
Name:Cleanroom Project
Floor:PVC
Temperature: HVAC system
Limit Discounts
Description
According to GMP regulations, the preparation, the fine drying package of API, the raw and auxiliary materials used in the preparation and the packaging materials directly in contact with the medicine should be produced in the clean area.
The clean room or clean zone of a pharmaceutical producing enterprise refers to the area that requires specified environmental control for dust particles and microbial pollution. The construction structure, equipment and use of the clean room can reduce the intervention, generation and retention of pollution sources in the area. Therefore, the clean room of a pharmaceutical producing enterprise has its own characteristics.
Characteristics 1.Clean room is a special designed room to exclude the particles in the air, bacteria and other harmful air pollutants 2. the indoor temperature, cleanliness, interior pressure, air velocity and air distribution, noise, vibration, and lighting, static control within the scope of a certain demand. 3. That is no matter how the outside air conditions change, its indoor all can maintain the original set requirements of cleanliness, temperature and humidity and pressure performance characteristic. |
Technical Parameters
| Suspended ceiling | Rock wool sandwich panel, Hollow glass megnesium sandwich panel, T-Grid ceiling, etc |
| Floor | Anti static PVC tile |
| Cleanliness | ISO5 to ISO8 |
| Project include | Structure: Wall Panel, Ceiling Panel, Windows, Doors, Various Fittings, Flooring HVAC: AHU, Ducting, Piping, Chiller, dehumidifier, etc. |
| Qualification Documents | IQ, OQ, PQ,DQ |
| Partition | Rock wool sandwich panel, EPS panel, Hollow glass megnesium sandwich panel, etc |
| Application | Biological engineeringLCM, LED, LCD, Electical product, CMOS Camera Module, Touch Play, Optical Module, FILM, Biological Pharmacy, Optoelectronic Display, PCB, Microelectronics, Dust-free coating, etc |
| Our Service | Professional Consultation, User-friendly English Sofaware, 24 hours technical support,Foreign installation and commissioning |
Installation
In order to know your requirement, please fill the following table carefully and supply CAD layout, so that we can provide correct scheme and quotation, thank you!
How to get a quotation >>






